Master scientific notation and significant figures with this concise guide, designed to simplify complex scientific calculations. Learn how to convert large numbers like 299,792,458 m/s or small ones like 0.000556 into scientific notation (e.g., 2.99792458 × 10⁸, 5.56 × 10⁻⁴) for precision in fields like chemistry and physics. Discover the rules for identifying significant figures, crucial for maintaining accuracy in calculations, such as rounding 25.6854 g + 0.17 g to 25.86 g. This 650-word article offers step-by-step conversions, practical examples, and a handy table to clarify processes. Boost your skills with practice problems and explore resources like ChemTeam for deeper learning.
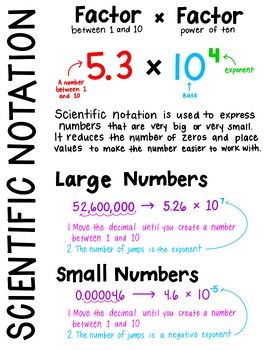
What Is Scientific Notation?
Scientific notation is a neat trick for writing really big or small numbers without drowning in zeros. It’s a must in fields like physics or chemistry, where precision matters. For example, instead of writing 299,792,458 m/s for the speed of light, you’d use 2.99792458 × 10^8. Cool, right?
Why Use Scientific Notation for Large and Small Numbers?
Scientific notation saves time and space. It’s perfect for:
By using scientific notation with significant figures, you keep your numbers manageable and precise.
Step-by-Step: Converting Numbers to Scientific Notation
Converting numbers to scientific notation is simple. Here’s how:
- Find the decimal point. For 234,999, it’s at the end (234,999.).
- Move the decimal until one non-zero digit is to the left (e.g., 2.34999).
- Count the moves. Moving left 5 times gives an exponent of 5: 2.34999 × 10^5.
- For small numbers, like 0.000556, move the decimal right until you get 5.56 (4 moves), so it’s 5.56 × 10^-4.
Quick Examples:
- 36,600 → 3.66 × 10^4 (moved left 4 times).
- 0.00416 → 4.16 × 10^-3 (moved right 3 times).
This method ensures scientific notation and significant figures work together to keep your numbers clear and precise.
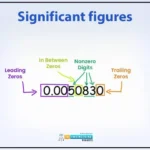
Understanding Significant Figures
Significant figures (or sig figs) tell you how precise a measurement is. When you measure 14.62 mL in a lab, those digits show what’s reliable. Significant figures in scientific notation help ensure your calculations don’t overstate precision.

Defining Significant Figures and Their Importance
Sig figs are the digits in a number that you can trust. They matter because:
- They reflect the accuracy of tools, like a ruler or scale.
- They prevent errors in calculations, especially in science.
- They pair perfectly with scientific notation to express measurements clearly.
For example, 300.16 has 5 sig figs, but 100 might have 1, 2, or 3—use scientific notation (e.g., 1.00 × 10^2) to clarify.
Rules for Identifying Significant Figures
Here are the rules for counting significant figures:
- Non-zero digits (1–9) are always significant. E.g., 123.4 has 4 sig figs.
- Zeros between non-zeros count. E.g., 2003 has 4 sig figs.
- Trailing zeros after a decimal (e.g., 300.16) are significant.
- Leading zeros (e.g., 0.00416) don’t count—only 3 sig figs here.
Table: Significant Figures Rules
| Rule | Description | Example |
| Non-Zero Digits | Always significant | 123.4 (4 sig figs) |
| Zeros Between Non-Zeros | Significant | 2003 (4 sig figs) |
| Trailing Zeros with Decimal | Significant | 300.16 (5 sig figs) |
| Leading Zeros | Not significant | 0.00416 (3 sig figs) |
These rules help you use significant figures with scientific notation accurately.
Applying Significant Figures in Calculations
When doing math with measurements, significant figures ensure your answer matches the precision of your data. The rules depend on whether you’re adding, subtracting, multiplying, or dividing.
Addition and Subtraction with Significant Figures
For addition or subtraction, match the number of decimal places in the least precise number.
Example:
25.6854 g + 0.17 g = 25.8554 g
- 0.17 has 2 decimal places, so round to 25.86 g (2 decimal places).
This keeps significant figures in scientific notation consistent with measurement precision.
Multiplication and Division with Significant Figures
For multiplication or division, use the number of sig figs in the number with the fewest.
Example:
2.1 × 4.5654 = 9.58734
- 2.1 has 2 sig figs, so round to 9.6.
Table: Significant Figures in Calculations
| Operation | Rule | Example |
| Addition/Subtraction | Match decimal places of least precise | 25.6854 + 0.17 = 25.86 |
| Multiplication/Division | Match sig figs of fewest | 2.1 × 4.5654 = 9.6 |
This ensures scientific notation significant figures reflect true accuracy.
Rounding Numbers in Scientific Contexts
Rounding is key when working with scientific notation and significant figures. It keeps your answers realistic and aligned with measurement precision.
General Rounding Rules for Scientific Accuracy
Follow these steps:
- Identify sig figs needed based on the calculation or measurement.
- Round up if the next digit is 5 or higher; round down if it’s 4 or lower.
- Check context: Exact numbers (e.g., 12 inches = 1 foot) don’t need rounding.
Example: 25.8554 rounded to 2 decimal places (for addition) becomes 25.86.
Rounding with Scientific Notation
When using scientific notation, round the coefficient to match sig figs.
Example:
2.34999 × 10^5 (6 sig figs) rounded to 3 sig figs becomes 2.35 × 10^5.
This ties significant figures with scientific notation to keep answers precise.
Practice Problems and Resources
Ready to test your skills? Let’s try some examples and point you to great resources for mastering scientific notation and significant figures.
Worked Examples for Scientific Notation and Significant Figures
- Convert to Scientific Notation:
- 36,600 → Move decimal 4 places left: 3.66 × 10^4.
- 0.0104 → Move decimal 2 places right: 1.04 × 10^-2.
- 36,600 → Move decimal 4 places left: 3.66 × 10^4.
- Count Significant Figures:
- 2003 → 4 sig figs (zeros between non-zeros count).
- 0.00000998 → 3 sig figs (leading zeros don’t count).
- 2003 → 4 sig figs (zeros between non-zeros count).
- Calculate with Sig Figs:
- (1.25 × 10^5) × (4.0 × 10^-2) = 5.0 × 10^3 (2 sig figs from 4.0).
- 14.62 mL + 0.1 mL = 14.7 mL (1 decimal place from 0.1).
- (1.25 × 10^5) × (4.0 × 10^-2) = 5.0 × 10^3 (2 sig figs from 4.0).
Where to Find More Practice and Tools
Want to keep practicing? Check out:
- ChemTeam (by John Park, Diamond Bar, CA) for awesome exercises.
- Online converters to practice scientific notation to significant figures.
- Resources recommended by educators like Dr. Masingale from Le Moyne College.
Try this: Search for “scientific notation calculator” to find tools that instantly convert numbers like 234,999 to 2.34999 × 10^5.
FAQs About Scientific Notation and Significant Figures
Q: How do I convert a number to scientific notation?
A: Move the decimal until one non-zero digit is to the left (e.g., 0.00563 becomes 5.63). Count the moves to get the exponent (e.g., 3 right = 10^-3). Result: 5.63 × 10^-3.
Q: Why are significant figures important in scientific notation?
A: They show how precise your measurement is, preventing overstated accuracy in calculations like lab results.
Q: What’s an exact number?
A: Numbers like 12 inches = 1 foot or 42 students in a class are infinitely precise and don’t affect significant figures.
Q: How do I round in scientific notation?
A: Round the coefficient to the desired sig figs (e.g., 2.34999 × 10^5 to 3 sig figs is 2.35 × 10^5).
Wrapping Up
Mastering scientific notation and significant figures makes handling numbers in science a breeze. Whether you’re converting 36,600 to 3.66 × 10^4 or rounding 25.8554 to 25.86, these tools keep your work precise and clear. Practice with examples, use online tools, and soon you’ll be crunching numbers like a scientist!